
IP NEWS IN JAPAN
The Japanese Patent Law and Examination Guidelines were partially revised last year. The revised Japanese Patent Law and Examination Guidelines are already in force except the revision of the JPO official fees and the introduction of the refund system for the fee for request for examination.
The Japanese Patent Law and Examination Guidelines were partially revised last year. The revised Japanese Patent Law and Examination Guidelines are already in force except the revision of the JPO official fees and the introduction of the refund system for the fee for request for examination.
I. Restructuring the patent-related fees
1. Revision of the JPO official fees (Article 195)
The official fees for JPO patent services have been revised for the purpose of correcting an imbalance in cost sharing among applicants and promoting appropriate actions in requesting examination.
By decreasing the application fee and patent annual fees and increasing the fee for request for examination, the number for requesting examination is expected to be decreased, while reducing the total cost per application/patent having 7.6 claims and paid up to the 9th year after issuance, which is an average one.
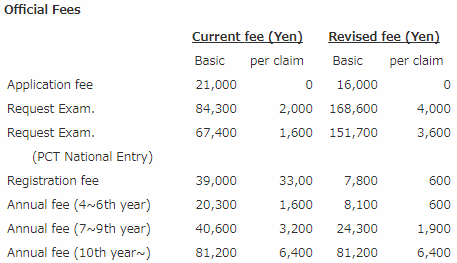
The revised application fee and examination fee will be applied to applications filed in Japan on or after April 1, 2004, and Japanese entries of PCT applications filed internationally on or after April 1, 2004. The revised registration fee will be applied to applications/patents for which a request for examination is filed on or after April 1, 2004.
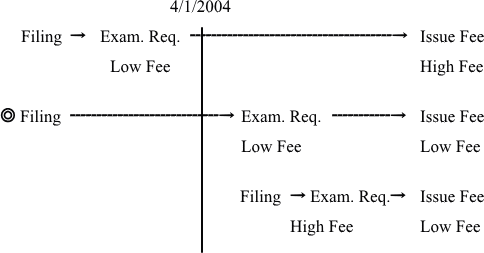
If request for examination is filed on or after April 1, 2004, issue fee and annual fees can be reduced.
2. Introduction of the refund system for the fee for request for examination
Article 195(2) of the Japanese Patent Law was amended to introduce the new refund system for the fee for request for examination.
It is currently allowed to withdraw a patent application but there is no provision to withdraw a request for examination. Even should an applicant withdraw an application after request for examination, the fee for request for examination is not refunded according to the current Law. Therefore, in case applicants lose the necessity for acquiring a patent after requesting examination, applicants are reluctant to withdraw their patent applications.
By introducing a system for refunding a half of the fee for request for examination, it is expected that applicants will withdraw their applications when they lose the necessity for acquiring a patent, resulting in a reduction of the JPO examination backlog. The new system provides applicants with the opportunity to save costs in such a case.
According to the new refund system, a half of the fee already paid for request for examination is refunded to the payer if:
the concerned application was withdrawn after the request for examination and before a first official action; and
the payer requested refunding within 6 months from the withdrawal of the application.
This new refund system will be effective as of April 1, 2004, and applied to applications withdrawn on and after October 1, 2003.
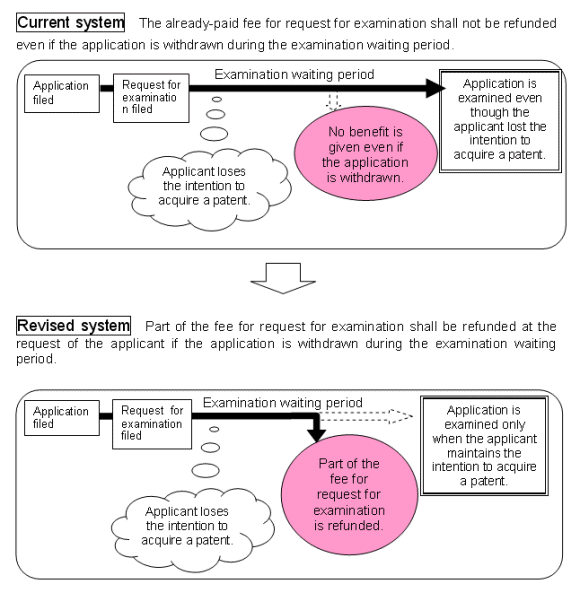
Excerpted from the JPO homepage
II. Integration of opposition and invalidation appeal systems:
Articles 113 through 120-6 of the Japanese Patent Law were deleted in order to abolish the opposition system.
In order to reduce the total time and parties’ burden required for opposing and invalidating patents, the coexisting opposition system and invalidation appeal (trial) system will be consolidated and integrated into a new invalidation appeal system. The new system was effective as of January 1, 2004.
To whom:JPO
By whom:anyone, no legal interest required
When:anytime, even after expiration of patent
Against what:any patent claim or any set of patent claims
Invalidation reason:novelty, obviousness, prior application, ?cjoint application, not assigner
Proceeding:inter partes(the patentee and the party who demanded a trial)
Law suit can be filed before the Tokyo High Court against trial decision:
Plaintiff/defendant are patentee/demandant, or vice versa.

Excerpted from the JPO homepage
III. Unity of invention:
Article 37 of the Japanese Patent Law and Article 25(8) of the Patent Regulations were amended as follows:
Amended Article 37 of Law:
Where there are two or more inventions, they may be filed in a single patent application provided that these inventions constitute a group of inventions complying with the requirement of unity of invention by having a technical relationship stipulated by the Patent Regulation.
Amended Article 25(8) of Regulation:
1.The “technical relationship” stated under Article 37 of the Patent Law shall mean a technical relationship in which two or more inventions are linked so as to form a single general inventive concept by having the same or corresponding “special technical features” among them.
2.The “special technical feature” provided in the former paragraph stands for a technical feature defining a contribution made by the invention over prior art.
3.The “technical relationship” provided in the first paragraph shall be examined, irrespective of whether the two or more inventions are described in separate claims or in a single claim described in an alternative form.
According to the old Law, unity of invention in Japan should be examined in a manner such that the main independent claim is selected as “the Specified Invention” and each of the remaining independent claims are examined as to whether they have any of the relationships stipulated in Article 37 of the Japanese Patent Law with respect to the Specified Invention. On the other hand, in the PCT research and examination stages, unity of invention is found when all claims are linked so as to form a single general inventive concept.
In order to improve harmonization with the PCT standard, the criteria for unity of invention (the scope of inventions that can be included in a single Japanese patent application) was revised.
Examples
– Product and another product having the same technical feature;
– Method and another method having the same technical feature;
– Product, and method of producing it and product and machine, instrument, device, the other for producing it;
– Product and method of using it;
– Product and another product solely utilizing specific properties of the Product;
– Product and method of handling it;
– Method and machine, instrument, the other used in the Method;
IV. Revision of Japanese Patent Examination Guidelines Regarding Medical Activity
1. The Japanese Patent Examination Guidelines regarding medical activity were revised as of August 7th, 2003. The revised Guidelines apply to all patent applications to be examined after August 7th, 2003, irrespective of their filing dates.
Summary of the revised Guidelines
Point 1) Methods for manufacturing medicines (such as hematological drugs, vaccines, genetically modified medicines) or medical parts (such as artificial bones, cultured epidermal sheets) utilizing material obtained from the human body are now patentable, even if such methods are performed on the presumption that the resultant manufactures will be returned to the same human body.
Point 2) Operating methods performed in a medical instrument or apparatus having no step that applies to the human body are now patentable.
2. Revised Examination Guidelines (Excerpt) (Added portions are underlined)
“Methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body” are deemed to be industrially inapplicable, and therefore unpatentable (Article 29-1 of the Patent Law).
Regarding Point 1)
Methods for treatment of samples that have been removed from the human body (e.g. blood, tissue, or hair), or methods of gathering data by analyzing the same, are not included in “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.” However, if the methods for treatment of these samples are performed on the presumption that they will be returned to the same human body (e.g. a treatment of blood by dialysis), then such methods are considered as “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.”
Methods for manufacturing medicines (such as hematological drugs, vaccines, genetically modified medicines) or medical parts (such as artificial bones, cultured epidermal sheets) utilizing material obtained from the human body are not considered “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body,” even if such methods are performed on the presumption that the manufactures will be returned to the same human body.
Furthermore, methods for contraception or delivery are also included in “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.”
Regarding Point 2)
An instrument or apparatus for use in such methods [medical acts], or a pharmaceutical substance, is a product, and is not included in “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.” On the other hand, an operating procedure on the human body by means of such an instrument (e.g. a scalpel) or a method for treating the human body with a pharmaceutical substance is considered, “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.”?@?@ Operations methods performed in a medical instrument or apparatus are not considered “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body.”
3. Examples
The following examples are considered as not consisting of “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body,” and are therefore patentable.
Example 1)
Title: Method for manufacturing cells for gene therapy
Claim:
A method for manufacturing cancer therapy cells, comprising:
introducing genes into W cells removed from the human body, using Z vector including DNA coding X protein and DNA coding Y protein.
Example 2)
Title: Image processing method in an X-ray CT device
Claim:
An image processing method in an X-ray CT device, comprising the steps of:
obtaining image data by performing reconfiguration processing based on dose distribution detected by X-ray detecting means;
comparing picture elements of said obtained image data with a threshold previously stored in a memory;
replacing the picture elements in a picture element area determined by the comparison to be lower than the threshold, with zero to remove noise;
and
transferring the image data clear of noise to display means.
Description:
An X-ray CT device according to the present invention can obtain excellent image data by being reconfigured based on dose distribution and removing picture elements lower than a threshold as noise. ?c?c
Explanation
A method invention expressing functions of a diagnostic device is considered substantially equivalent to the diagnostic device itself as long as the recited steps are all performed in the device, and therefore such a method invention is not considered as one of “methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body”, that is patentable.
However, if a claim describes a step for detecting a signal from the human body or a step for applying to the human body, then such a claim is considered a diagnostic method on the human body or a preparative treating method and is therefore unpatentable.
4. Patentable and unpatentable medical inventions according to the revised Guideline are listed below for your reference:
Unpatentable medical inventions:
Methods for treatment of the human body by surgery or therapy and diagnostic methods practiced on the human body (medical act), including (without limiting sense):
* Operating procedures on the human body by means of a medical instrument;
* Methods for treating the human body with a pharmaceutical substance;
* Methods for contraception or delivery;
* Methods for surgical operations and drawing blood;
* Cosmetic methods for surgical operations even if they have no therapeutic or diagnostic purpose;
* Methods of giving or injecting medicine, or giving physical treatment to a patient for cure or restraint of a disease;
* Methods of transplanting or implanting substitute organs such as artificial internal organs or artificial limbs;
* Methods of preventing a disease;
* Methods of treatment for the maintenance of physical health;
* Preparatory methods of treatment by therapy, auxiliary methods for improving treatment results, or methods for nursing associated with the treatment;
* Methods of gathering various kinds of data by measuring structures or functions of an organ in the human body for medical purposes such as detecting diseases or recognizing or judging the physical condition of the human body, or methods of judging the condition of diseases based on the data; and
* Methods of measuring the shape or size of internal organs or the conditions of the interior or exterior of the human body for medical purposes of detecting diseases or recognizing or judging the physical condition of the human body.
Patentable medical inventions:
* Medicines;
* Medical instruments or apparatuses;
* Methods for treatment of samples that have been removed from the human body (e.g. blood, urine, tissue, or hair), and methods of gathering data by analyzing the same;
* Operating methods performed in a medical instrument or apparatus having no step for applying to the human body; and
* Methods for manufacturing medicines (such as hematologic drugs, vaccines, genetically modified medicines) or medical parts (such as artificial bones, cultured epidermal sheets) utilizing material obtained from human parts (see the central large box in Figure shown below).
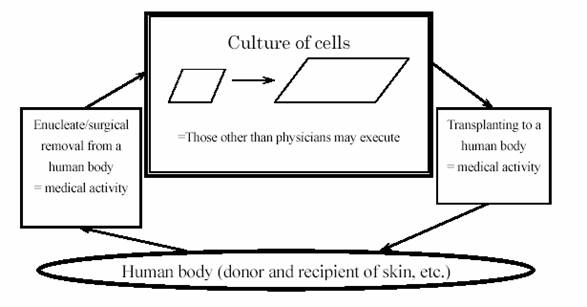
V. Japanese Patent Attorney can represent his client in IP infringement lawsuit
In the past, only general lawyers (attorneys at law) were allowed to represent their clients in IP infringement lawsuit and patent attorneys were able to only assist general lawyers before the court. However, most general lawyers have insufficient technical background to understand and handle high-tech cases. Then, the Japanese Patent Attorney’s Law was revised so as to allow patent attorneys satisfying the following requirements to represent clients jointly with general lawyers before civil courts for IP infringement cases regarding patent rights, utility model rights, design rights, trademark rights, semiconductor mask work rights and some rights based on the Unfair Competition Law.
Requirements:
– Completed Special Training Course; and
– Passed the Examination for the Qualification.


